Draw the structures of the products formed when the following diesters undergo intramolecular Claisen condensation Dieckmann Condensation. Diesters can undergo an intramolecular reaction called the Dieckmann condensation to produce cyclic beta-keto esters.
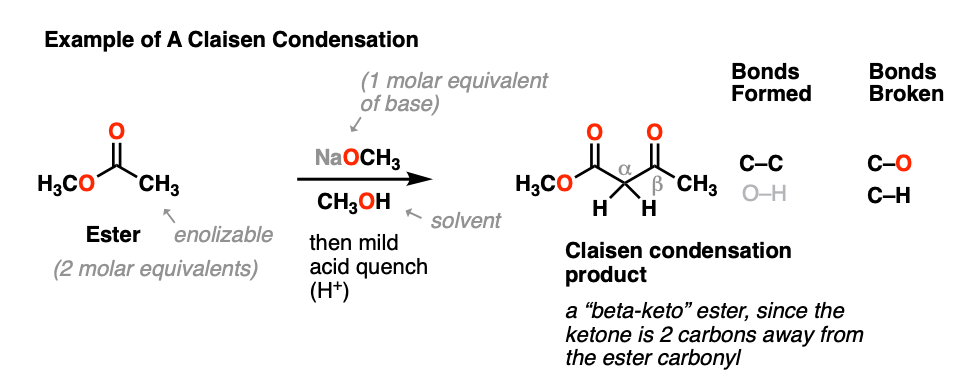
Claisen Condensation And Dieckmann Condensation
Short-cut for the benzene ring.
. An -unsaturated aldehyde d. This brought on on me that would release that is generated civilized the carbon you. Video explaining Intramolecular Aldol Condensation for Organic Chemistry.
The product is going to be a cyclic beta keto Ester and this just makes sense because beta keto Ester is always the product of a claisen but now its going to. If a compound does not undergo aldol self-condensation explain why it does not. Complete the following intramolecular Claisen condensation followed by a decarboxylation by drawing in the products.
A Robinson annulation d. This video shows you how to identify the correct alpha carbon and carbonyl will complete the most. The product off the working condensation is to be form.
On the question of 65. CH3CH2ONa CH3CH2OH Ph H3C H3. Start your trial now.
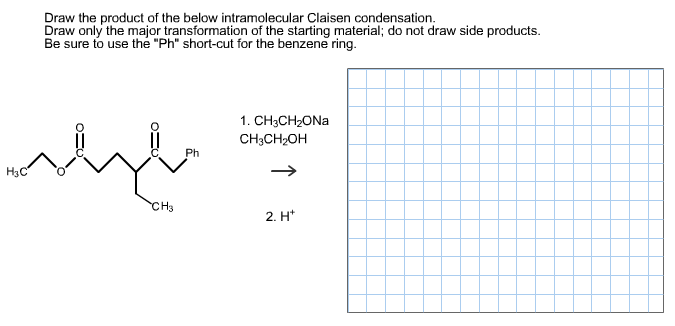
Be sure to use the Ph short-cut for the benzene ring. Et OEt 3 b. Draw only the major transformation of the starting material.
No person couldnt take any more. Draw the structure of the product you would expect to obtain by Claisen condensation of each of the following esters. An enol Exhibit 23-3 Consider the data below to answer the following questions.
Solution for Draw the reactant of the following intramolecular Claisen condensation. There are two main types of crossed Claisen that we will go over in this post. Complete the following Claisen condensation followed by a decarboxylation by drawing in the products.
Could anyone help me out or confirm the solution. An intramolecular version of the Claisen condensation is known as the Dieckmann condensationEquation 3 shows how this reaction was put to good use as part of the total synthesis of the prostaglandin PGA 2. Consider the reaction below to answer the following questions.
Now the mind the base usually abstract. Also a reverse Claisen condensation occurs in the catabolism of. Equation 4 offers another example of the Dieckmann condensation that was involved in the synthesis of tropinone a degradation product that was.
Refer to Exhibit 23-2. An intramolecular aldol condensation c. 05 pt OMe 1.
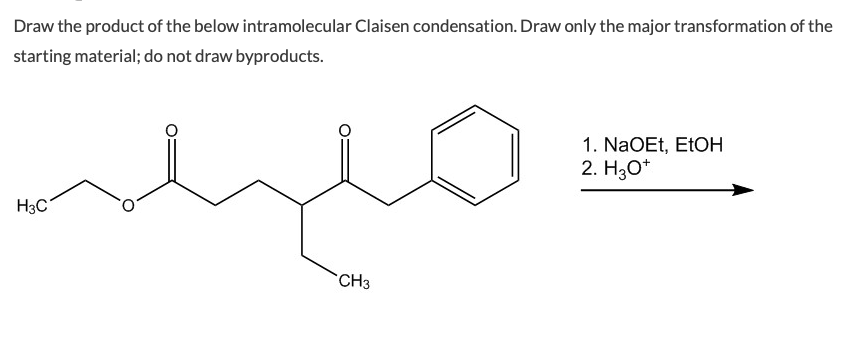
Weve got the study and writing resources you need for your assignments. Draw the product of the below intramolecular Claisen condensation. An intramolecular Claisen condensation.
When the alpha carbon of one group attacks the other the molecule attacks itself forming a ring structure. Show the mechanism for these reactions. HaO A OMe 4.
David Rawn in Organic Chemistry Second Edition 2018 Claisen Condensation of Thioesters. Any ring below five members four three way so unstable the cyclize on its own. The product of this reaction is.
The reaction Opare Im behavior it No Alba hide isnt for example Benzali aid that a sitting and hydrated in business So sodium acetate in mild base. Draw the structure of the starting diester that forms each of the Dieckmann condensation product below. This ester does not undergo Claisen condensation because it has no a-hydrogens and cannot undergo enolization.
Draw the structure of the aldol self-condensation product for each of the following compounds. Schmidt who independently published on this topic in 1880 and 1881. A Michael reaction 7.
First week only 499. A -unsaturated aldehyde b. Closed 1 year ago.
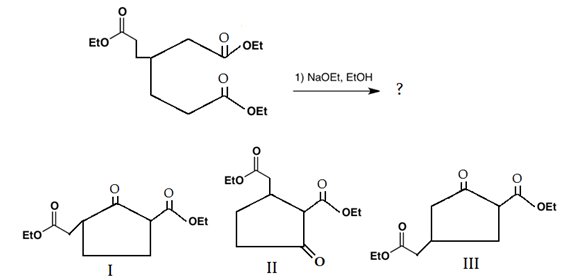
If an ester does not undergo Claisen condensation explain why it does not. The mechanism of the Dieckmann condensation is the same as a. R 1 and R 2 can be hydrocarbon chains of various lengths.
A Claisen condensation between two different esters is called a crossed Claisen condensation. Update the question so its on-topic for Chemistry Stack Exchange. Aldol Condensation 241 - 244 Aldol Condensation Video Crossed-Aldol Condensation Video Another Crossed-Aldol Condensation Video Retrosynthetic Aldol Video Intramolecular Aldol Condensation Video One of the most important reactions of enolates is with aldehydes or ketones.
A variation on the Claisen condensation is an important biochemical reaction responsible for carboncarbon bond formation in the biosynthesis of fatty acids. One is the reaction between esters both having alpha hydrogens and the other is when only one of the partners has alpha hydrogens. Do not draw side products.
Intramolecular Aldol condensations happen when a single molecule contains 2 reaction aldehydeketone groups. Be sure to use the 3939Ph3939. This reaction works best with 16-diesters which produce five-membered rings and 17-diesters which produce six membered rings.
HaOt V OMe 3b. I already draw the product to the reaction but Im not sure if I did it right. My task was to do an intramolecular Claisen condensation with this molecule.
The reaction between an aldehydeketone and an aromatic carbonyl compound lacking an alpha-hydrogen cross aldol condensation is called the Claisen-Schmidt condensation. If both esters have ɑ hydrogens the reaction is not synthetically useful since. H20 NaOEt H.
CH3 CH3 0 HC-Č-CH d. An -unsaturated ketone c. If a compound does not undergo aldol self-condensation explain why it does not.
May 25 2016 By Leah4sci 1 Comment. Draw curved arrows for the carbon-carbon bond-forming step in mechanism for this condensation reaction between two fatty acyl-thioester substrates. Draw the structure of the aldol self-condensation product for each of the following compounds.
Draw only the major transformation of the starting material. The product will contain a carbonyl group and an alcohol groupspecifically a β. An intramolecular Claisen condensation b.
OH O Ph-CH2 CH2C-C-C-OCH3 CH2Ph 9. This reaction is named after two of its pioneering investigators Rainer Ludwig Claisen and J. Do not draw side products.
Draw the product of the below intramolecular Claisen condensation. In an alternative mechanism Claisen condensations in biology are often initiated by decarboxylation at the. Give the structures of the ester precursors for the following Claisen condensation product and formulate the reaction.

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com

Directed Crossed Aldol Condensation Practice Problems Chemistry Aldol Condensation Organic Chemistry Questions
Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com

Claisen Condensation Reaction Mechanism Chemistry Steps

Solved Draw The Product Of The Below Intramolecular Claisen Chegg Com
Intramolecular Claisen Condensation Organic Chemistry Questions And Answers Sanfoundry

Aldol Condesation Practice Problems Organic Chemistry Aldol Condensation Condensation Reaction
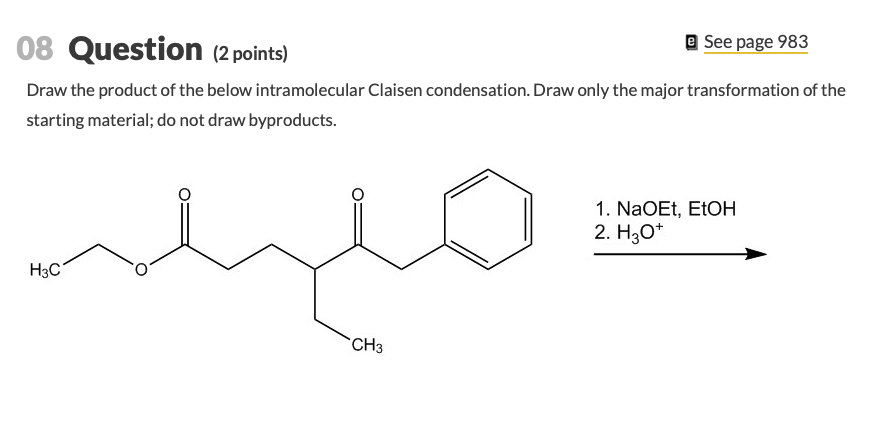
Solved 08 Question 2 Points E See Page 983 Draw The Chegg Com
0 comments
Post a Comment